Feb 15 2022. This would be known as the activated complex.

Cell And Molecular Biology Concepts And Experiments Karp Cell And Molecular Biology Gerald Karp 9781118206737 Am Molecular Biology Molecular Experiments
The activation energy of a reaction can be altered by the addition of A substrates D ATP B heat energy E ADP C enzymes 1421.

. Lighting a match is a great example of using activation energy. The heat that was generated could then produce enough activation energy to allow the chemicals on the match react and ignite into flame. Which phrase defines activation energy.
The energy threshold that must be reached before a reaction can proceed and products may be formed. 1energy input needed to break bonds of reactants. Activation energy is best described as ___ the energy required to initiate a chemical reaction Rank the grades of coal by their relative desirabilities starting with mos desirable at the top.
High-energy molecule with strong bonds and a stable structure. The activation energy units are LCalmo KJmol and Jmol. Specifically the higher the activation energy the slower the chemical reaction will be.
The structure of a. In transition-state theory the activation energy is the difference in energy content between atoms or molecules in an activated or transition-state configuration and the. Activation Energy is the energy which must be provided to potential reactants.
Catalysts affect reaction rates by. 3White phosphorus has a lower activation energy than red phosphorus. But it is very important to know which types of reactions require activation energy and how much.
D the energy level of the products. ClNO 2 g NO g NO 2 g ClNO g. Moreover in a chemical or a nuclear system so that a spark can be given to a chemical reaction or a nuclear reaction.
Energy of activation The energy of activation is best described as Multiple Choice the speed at which a reaction proceeds to form products. The minimum amount of additional energy needed by a reacting molecule to get transformed into the product is termed activation energy. This is because molecules can only complete the reaction once they have reached the top of the activation energy barrier.
Up to 24 cash back 2. A the energy level of the reactants. Questions transer between systems quick check 1.
Energy stored in chemical bonds d. This can be understood by turning once again to the reaction between ClNO 2 and NO. Activation energy is the minimum quantity of energy that the reacting species must possess in order to undergo a specified reaction.
A protein that speeds up a chemical reaction. Activation energy in chemistry the minimum amount of energy that is required to activate atoms or molecules to a condition in which they can undergo chemical transformation or physical transport. Activation Energy Activation Energy The Activation Energy of Chemical Reactions Only a small fraction of the collisions between reactant molecules convert the reactants into the products of the reaction.
4Products have higher chemical potential energy than reactants. 5Higher activation energy results in a slower reaction rate. Well the activation energy is the extra energy given to get useful work done.
2energy stored in chemical bonds. Activation energy can be described as the a. Activation energy can best be described as.
Activation energy is the minimum amount of energy needed to start a chemical reaction. B the difference in energy between reactants and products. Group of answer choices a The amount of energy lost in an exothermic reaction.
Energy required to break a chemical bond b. E the maximum energy level of the reaction. Energy required to break a chemical bond b.
We generally denote this energy by E. None of the answer choices are correct. Striking a match against the match box helps to create friction which in turn creates heat.
The activation energy of a chemical reaction is closely related to its rate. In chemistry we call it the minimum amount of energy or threshold energy needed to activate or energize molecules or atoms to undergo a chemical reaction or transformation. It is the required to form or break the bonds of reactant molecules that results in a chemical reaction.
So the answer. Energy output when product bonds are formed c. The Activation Energy can best be described as.
Energy input needed to break bonds of reactants 2. C the difference in energy between reactants and the maximum energy.

100 Original Thailand Ointment Rhinitis Mint Nasal Refresh Cream In 2022 Ointment Allergic Rhinitis Nasal Congestion
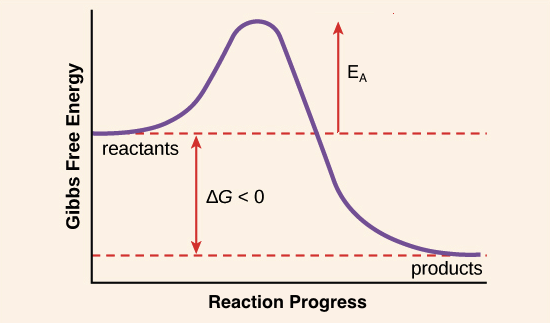
Activation Energy Article Khan Academy

Achieve Higher Grades With Qwivy Com In 2021 Ekg Interpretation Question And Answer Interpretation
0 Comments